The ratio of rates of diffusion of gases X and Y is 1:5 and that of Y and Z is 1:6. The ratio of rate of diffusion of Z and X is:

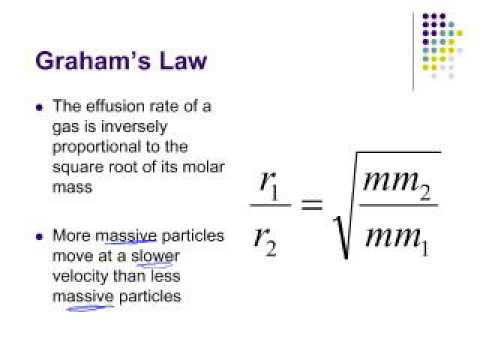
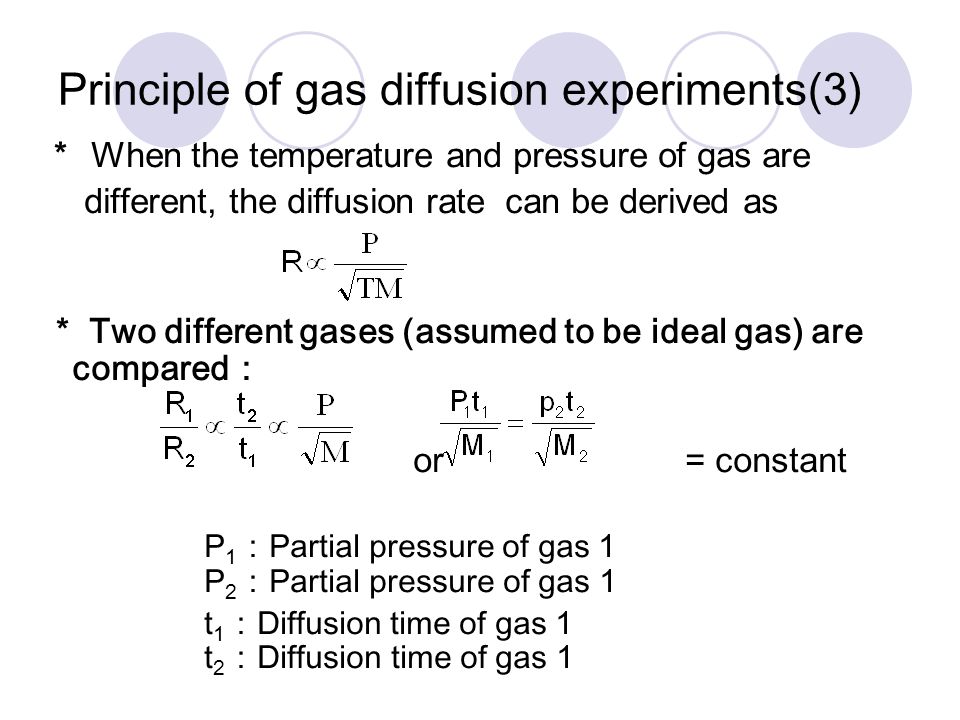
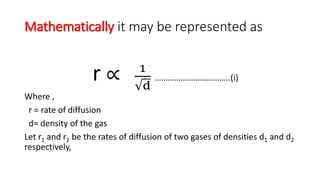
SOLVED:The rate of diffusion of a gas is proportional to: (a) (P)/(√(d)) (b) (P)/(d) (c) √((P)/(d)) (d) (√(P))/(d)

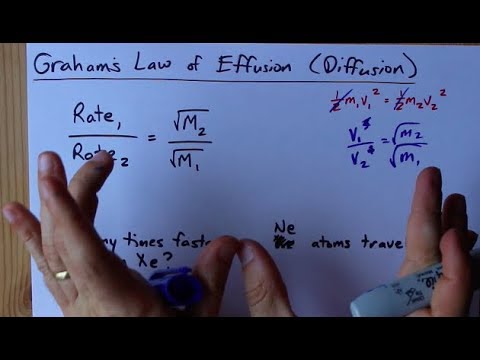
Rates of diffusion of two gases A and B are in the ratio 2:1. If the molecular mass of gas A is 16g. Find the...
The rate of diffusion of two gases X and Y is in the ratio of 1:5 and that of Y and Z in the ratio of I: 6. The ratioof the rate

SOLVED: In this experiment, we will measure the diffusion rates of hydrogen chloride gas (HCl), ammonia gas (NH3), and ammonium gas (NH4) obtained from hydroxide and hydrochloric acid solutions. When these gases




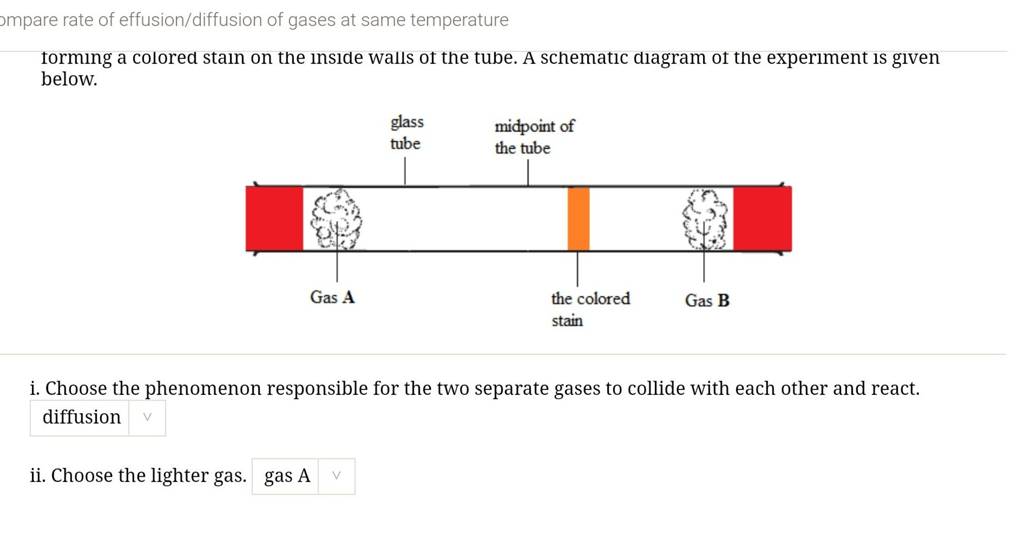

![ANSWERED] SolveLancer Test The rate of diffusion of... - Physical Chemistry ANSWERED] SolveLancer Test The rate of diffusion of... - Physical Chemistry](https://media.kunduz.com/media/sug-question-candidate/20210625230951982656-1884508.jpg)